This Department of Energy (DOE) project titled “Experimental and Computational Studies of Crystal Nucleation in Composition Gradients” aims to build on our previous work and established capability using nanocalorimeters to study intermetallic reactions. This project is a continuing collaboration with Dr. David LaVan of the National Institute of Standards and Technology. Nanocalorimeters are small, microfabricated devices which enable rapid heating and precise thermal measurement of small samples.
In previous efforts [1], extremely sharp gradients in crystalline Al/Ni multilayers provided a valuable system for studying nucleation conditions due to the precision control of the thermal history. Isochronal We now apply the same processes to binary metallic glass-forming systems, allowing for a broader control of the gradient. Our initial study [2] used Cu-Zr as the binary glass-forming system, but the gradients produced were not sufficiently steep to retard nuclation during isochronal heating.
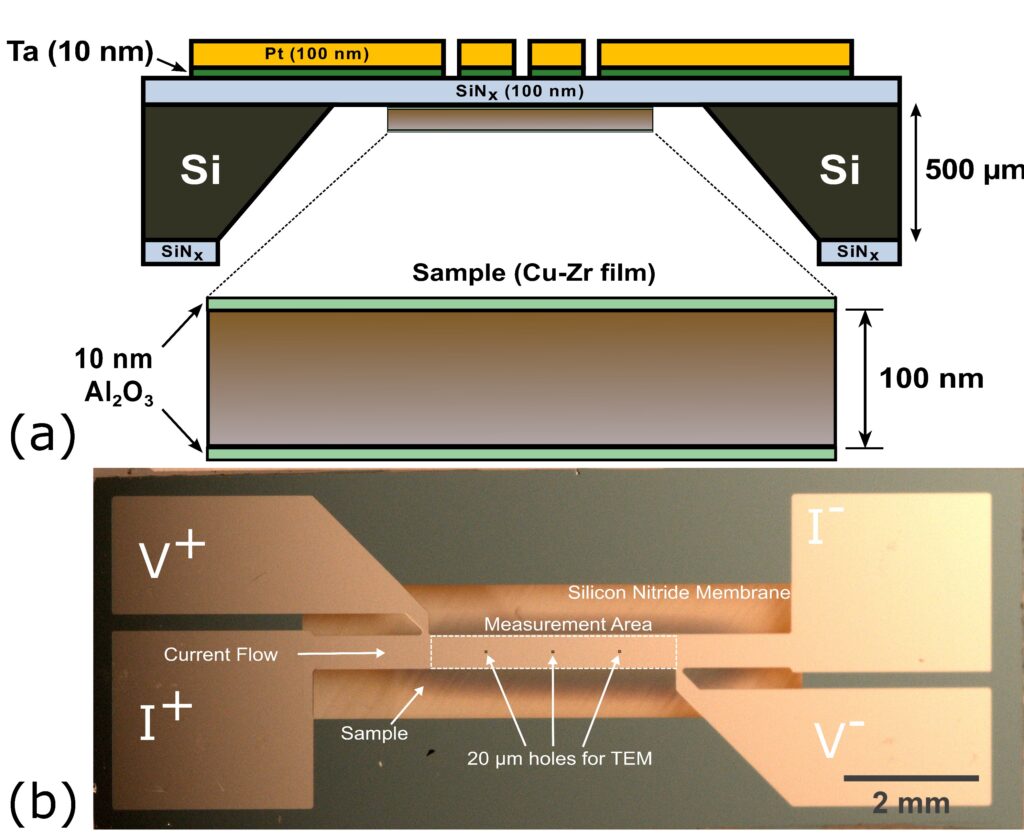
The experimental component of the project studies the roles of concentration gradient on nucleation rates and temperatures using isochronal and isothermal nanocalorimetry. Isothermnal nanocalorimetry will be performed in situ in a TEM to measure nucleation rates, using a new nanocalorimeter design which incorporates holes in the silicon nitride membrane to reduce background signal.
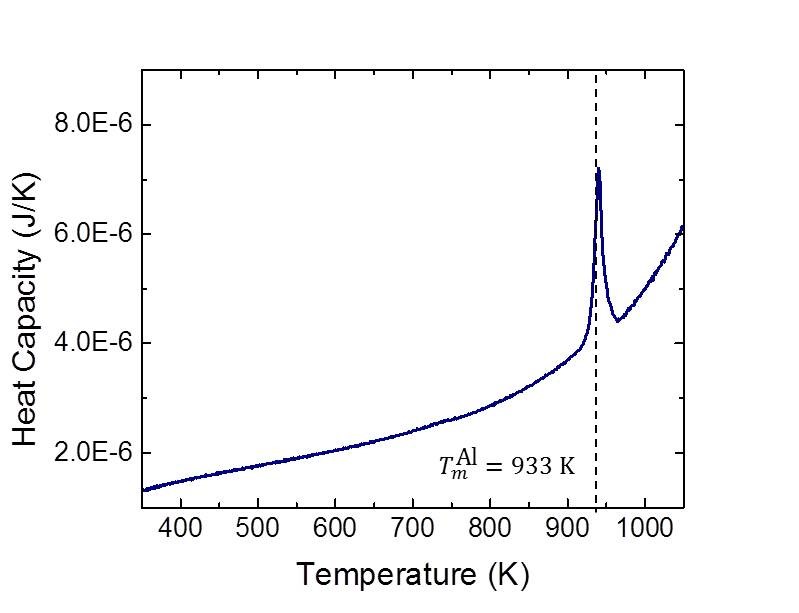
The experimental work is coupled with molecular dynamics simulations and analytical modeling efforts aimed at predicting nucleation in steep concentration gradients. These simulations are performed by our collaborator, Prof. Michael Falk , and his laboratory. The modeling efforts to date have included predictions of both heterogeneous [3] and homogeneous [4] nucleation within steep composition gradients in the Al/Ni system. Future efforts will model the same material systems explored experimentally. The theory developed in the project will be a notable improvement on our existing understanding of how nucleation occurs in sharp concentration gradients, with the experiments representing the first rigorous and systematic verification of such a model.
References
Grapes, M. D.; Santala, M. K.; Campbell, G. H.; Lavan, D. A.; Weihs, T. P. A Detailed Study of the Al3Ni Formation Reaction Using Nanocalorimetry. Thermochim. Acta 2017, 658, 72-83.
Arlington, S. Q.; Yi, F.; Lavan, D. A.; Weihs, T. P. A Nanocalorimetric Study of the Effect of Composition Gradients on Crystallization in Amorphous Cu-Zr Thin Films. AIP Adv. 2019, 9 (3), 035324.
Yi, P.; Falk, M. L.; Weihs, T. P. Intermetallic Formation at Deeply Supercooled Ni/Al Multilayer Interfaces: A Molecular Dynamics Study. J. Appl. Phys. 2018, 124 (16), 5303.
Yi, P.; Ruan, D.; Weihs, T. P.; Falk, M. L. Predicting the Rate of Homogeneous Intermetallic Nucleation within Steep Composition Gradients. J. Phys. Chem. C 2020.
