This project, funded by the Defense Threat Reduction Agency, is focused on developing a combination of reactive fuel materials with biocidal and chemical neutralization capabilities. The aim is to develop reactive composites with tunable ignition and combustion performance that enable a combination of thermal and/or chemical inactivation of biological/chemical agents over a range of timescales and relevant length scales during a blast. The approach involves the use of multiple metals in composite powder structures. The composite powders offer mixing on nano to micrometer lengthscales, and release of large amounts of heat which can partially or fully melt the particles. When heated, the powders react at low temperatures yet are able to oxidize in air and burn at higher temperatures with greater efficiencies than their corresponding commercial counterparts
Recently, most of our research efforts have shifted towards chemical warfare agents (CWA) with the launch of the Materials Science in Extreme Environment University Research Alliance led by The Johns Hopkins University. This project focuses on improving our ability to neutralize CWA simulants such as Di-isopropyl Methyl-Phosphonate (DIMP) utilizing composite metal powders synthesized in our laboratory. These powders have previously shown efficacy towards defeating bio-agent such as anthrax, and preliminary studies demonstrate their potential towards chemical-agent defeat applications.
Below is a slow motion video of reactive particles mixing with HIO3, which decomposes into biocidal iodine gas.
Experimental Combustion Studies
Our lab is designing and building a combustion chamber to perform experimental combustion studies. That chamber will include the following capabilities:
- 3-color pyrometry and SHEAR analysis to determine the temperature and species of the burning particles
- Monitoring temperature of the surrounding gaseous environment using NIR laser spectroscopy
- High speed videography for ignition and combustion propagation
- LWIR Laser spectroscopy to monitor chemical agent simulant concentration
These diagnostics aim to explore the role of composite metal powders in CWA simulant neutralization by characterizing reaction profiles and mechanisms from a thermal property standpoint, as well as chemical properties. Beyond chemical-agent defeat, this chamber provides the ability to study combustion behavior over longer distances. The chamber will utilize a non-thermal plasma ignition system which will allow for a better understanding of the particle reactivity in chemical-agent defeat or combustion studies without the large pressure or thermal input associated with traditional explosive-charge or flame ignition methods.
The modular combustion chamber provides a wide range of experimental capabilities. Initially these will aim to address how we can tune composite metal powders to enhance chemical agent defeat from both a thermal-kill and chemical-kill perspective. By using burning metal particles to interact with and neutralize chemical-agent simulants, we are not only exposing simulants to elevated temperatures, but also generate a physical interaction between the burning metal particles, combustion by-products (such as nano-oxides), and the simulant of interest. Understanding this interaction will allow us to tune the chemistry and stoichiometry of composite metal powders for enhanced chemical agent defeat.
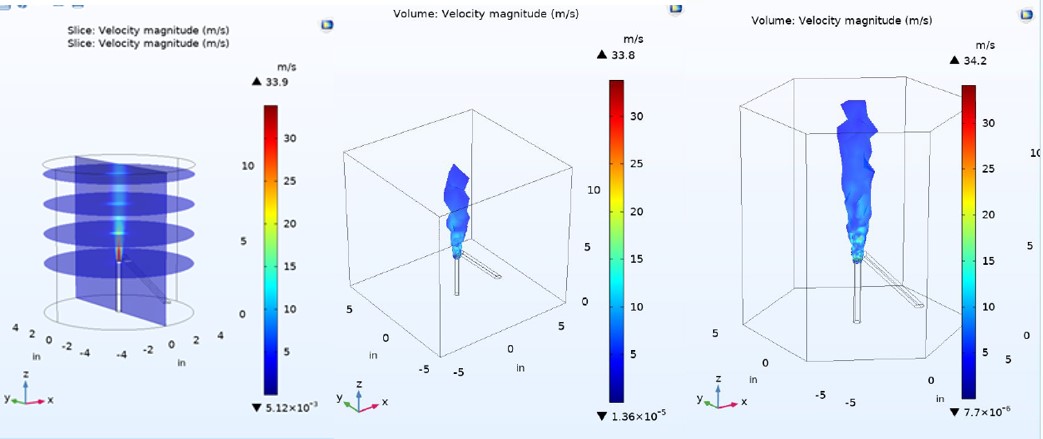
CFD Modelling for Modular Combustion Chamber
Using COMSOL Multiphysics, the group actively determines the concentration, velocity and temperature profiles for the combusting media inside the combustion chamber with varying design configurations. The CFD model will be used in conjunction with preliminary experimental configurations of the chamber to describe the combustion environment of particles ignited by a plasma as well as the proposed concentration profile of the simulant throughout the chamber due to the inlet flows. The scope of the model involves the use of steady-state and (in future) time dependent models to determine the mixing zones and proposed areas of interest for observing degradation of the chemical agent. The approach is to use Turbulent flow models to describe mixing zones and to couple these physics with Heat transfer and reacting mass transfer or chemical species transport equations. The model will be validated by experimental studies determining particle velocimetry and temperature profiles.
Non-Traditional Thermites
Non-traditional thermites provide a focused, condensed phase reaction resulting in the production of almost no gaseous products. These formulations provide a covert method for operational use of thermite in sensitive situations. Current research is aimed at improving reaction rates by evaluating the fuel/oxidizer particle contact. Reactive, Al-Zr composite powders are prepared using arrested reactive ball milling (ARM) to generate varying atomic ratios of the metal fuel components. Detailed microstructure information will be determined using SEM, DTA, PSA, pycnometry, and XRD. Ignition temperature and burn rates will also be determined for the thermite formulations.

