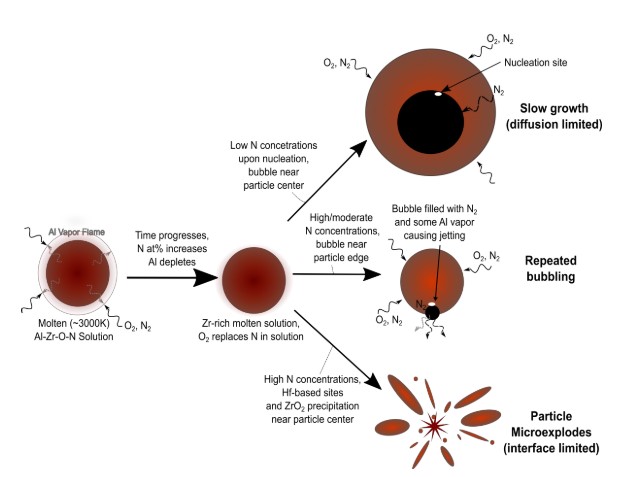
The contribution of metal powders to propulsion, blast, and thermobaric effects relies on their ease of ignition and efficiency of combustion. However, there is still limited knowledge of the reactions that occur on the microsecond and millisecond timescales when metal powders ignite and combust in air, as well as the more complex, composite derivatives that arise. Of the metal powders commonly used in energetic formulations, Al and Mg combust primarily in the vapor phase by first evaporating and then oxidizing, while Ti and Zr combust in a condensed liquid or solid phase and are reactive with nitrogen, not just oxygen. We consider this type of reaction to be dual-phase combustion. Combining elements from these two groups can leverage both modes of combustion simultaneously to enhance burn rates and efficiencies for a given powder size.
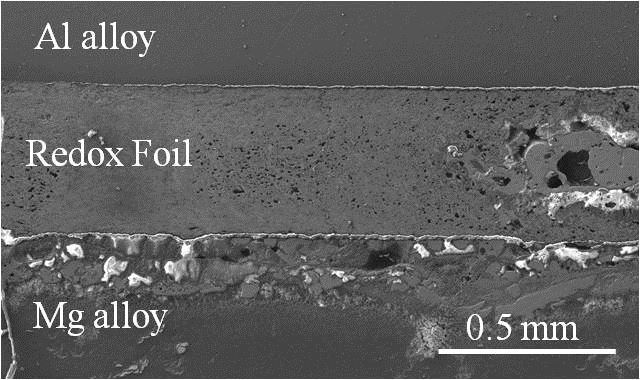
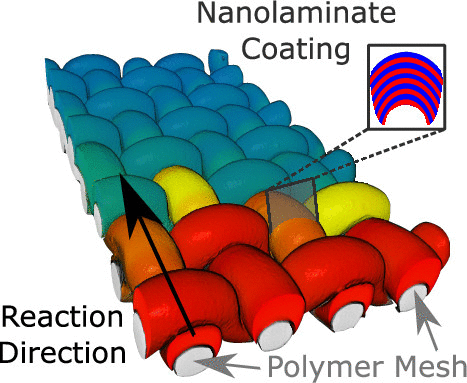
The Weihs Group actively conducts research to design, tune and characterize materials comprised of metals, alloys, oxides, and metal composites for reactions that span a wide range of applications. These include neutralizing harmful toxins or chemical agents and biological agents, chemical time delays, non-traditional thermites, and structural reactive materials. The materials are typically nanocomposites with tuned microstructures prepared by high energy mechanical ball milling, magnetron sputtering, swaging or rolling. To aid in understanding the complex reaction mechanisms involved in optimized material performance, we develop novel experimental diagnostics and utilize machine learning algorithms for data mining and tuning material selection.